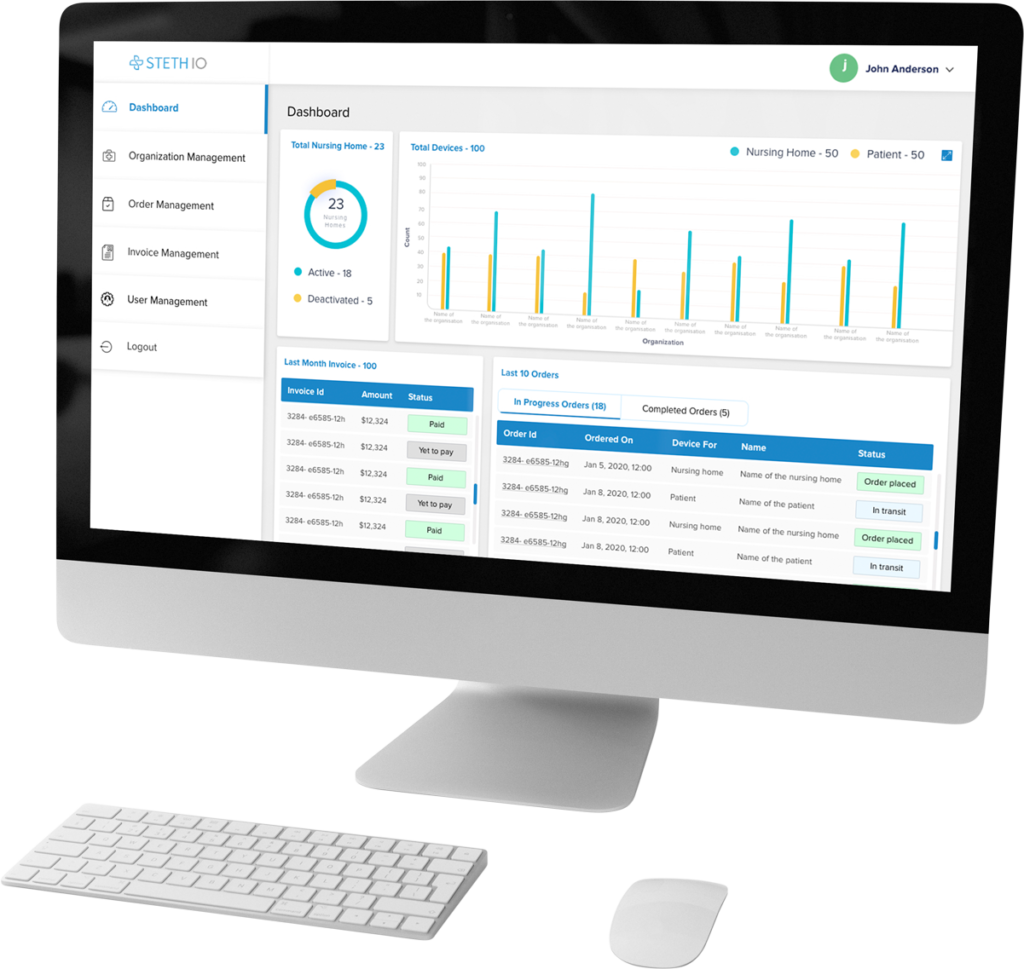
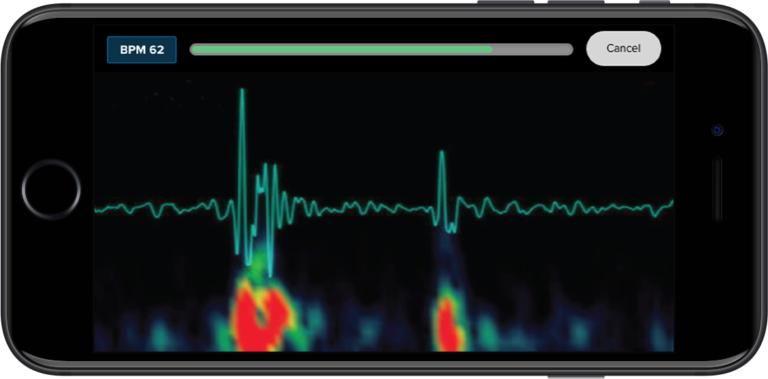
Screen, monitor, and document clinical trial progress with a single telemedicine and remote patient monitoring platform.
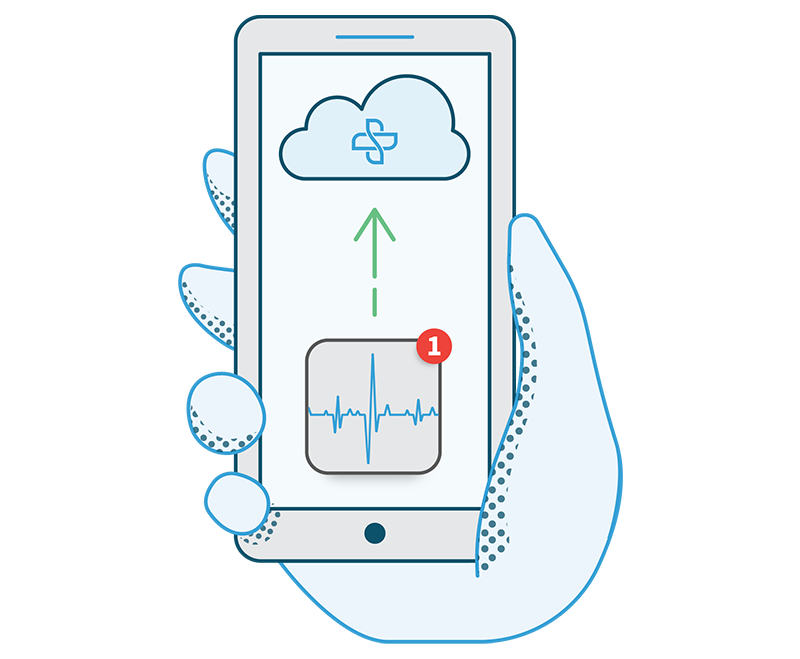
Steth IO provides an all-in-one telemedicine platform for enrolling, screening, and monitoring patients participating in clinical trials. Our system is designed to simplify clinical trial data capture—recording and storing patient documentation for reference and tailoring patient-provider communication to match the cadences determined by the trial protocol.
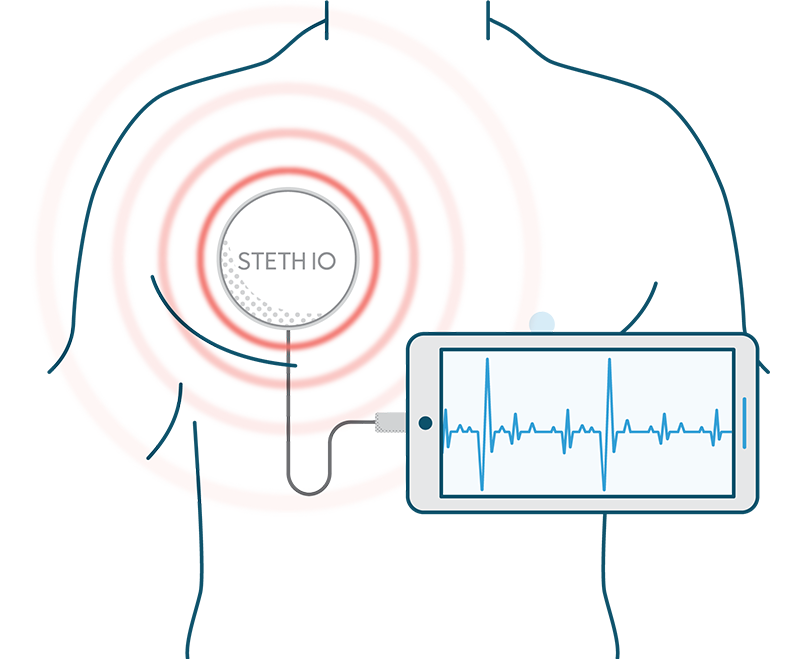
Because Steth IO offers next-generation telemedicine capabilities—including live heart and lung sound examination—remote participant experience becomes akin to an in-person visit. As such, clinical trials can be expanded beyond traditional geographical boundaries for wider study and greater participation.